Allanite Rare Earths Processing Breakthrough: Successful Completion of Impurity Removal Neutralization Tests
Highlights
-
Impurity Removal Metallurgical Tests Yield Allanite Rare Earths Processing Breakthrough:
- Impurity removal is one of the last steps in the hydrometallurgical processing of rare earths elements (“REE”) and is performed to remove non-REE minerals from the leach liquor prior to solvent extraction and separation (i.e. the final steps before producing rare earths oxide). Historically, this has been a challenging step for processing allanite-based REEs, like Halleck Creek’s ore, as the mineral typically produces unwanted byproducts such as gypsum and silica gel, resulting in additional and difficult processing steps to remove them.
- In a recent and extensive impurity removal test program on Halleck Creek ore minimal gypsum and silica gel were formed during the process, which points to immense operating benefits, including but not limited to the reduction of rare earths yield loss and fewer processing steps resulting in potentially lower capital and operating expenses.
- These results de-risk what has historically been a material technical and economic hurdle in the processing of allanite-based rare earths elements (i.e. Halleck Creek’s ore) and represent a major milestone in unlocking Halleck Creek’s vast REE supply potential.
-
Adverse Elements removed from Leachate Solutions
- Effectively 100% of iron, titanium and other deleterious elements precipitated from Leachate Solutions
- Over 99% of silica and aluminum precipitated from Leachate Solutions
- Magnesium Oxide (MgO) chosen as the optimal neutralizing reagent
DENVER, Oct. 13, 2025 (GLOBE NEWSWIRE) -- American Rare Earths (ASX: ARR | OTCQX: ARRNF | ADR: AMRRY) (“ARR” or the “Company”) has successfully completed a critical stage in its mineral processing program, the first phase of impurity removal testing, with highly encouraging results. This milestone confirms that key contaminants like iron, aluminum, silica and others can be effectively removed from Halleck Creek ore, paving the way for efficient rare earths extraction. Importantly, the tests showed minimal formation of problematic by-products like gypsum and silica gel, a common challenge in processing allanite-based rare earths elements.
SGS completed the neutralization testing at their laboratory in Lakefield, Ontario, Canada. The results will be a key input in the hydrometallurgical processing portion of the Pre-Feasibility Study flowsheet. The objective of impurity removal is to remove deleterious elements (such as iron, aluminum, silica and others) from the rare earth elements (“REE”) in leachate solutions. Impurity removal is the next processing step after leaching1 and is accomplished by adding reagents to neutralize the leach liquor at specific pH ranges. At different pH levels, the various deleterious elements precipitate out and are removed from the leach solution by filtration. Throughout this robust testing program, ARR’s third-party lab, SGS tested various reagents over various pH ranges to determine the optimal conditions for Halleck Creek. The next step of hydrometallurgical testing will be to create a mixed rare earth oxide, which is a precursor to solvent extraction and creating individual, separated oxides used in permanent magnets.
Six potential neutralizing agents were tested on REE enriched leach solutions. Magnesium oxide (MgO) and magnesium carbonate (MgCO3) yielded the best results. Looking forward, magnesium oxide is a more cost-effective reagent than magnesium carbonate and was selected as the optimal neutralizing reagent for the mineral processing flow-sheet.
Given allanite (i.e. REE host mineral) is rich in calcium and silica, it was anticipated that gypsum (i.e. calcium sulfate) and/or silica gel might form during the impurity removal test program. The solutions neutralized with MgO formed few of these unwanted products, which will likely yield significant operational benefits, including but not limited to reduction of REE yield loss, fewer additional processing steps and lower capital and operating expenses. Historically, the formation of these products has proven to be a material technical and economic hurdle to overcome in the processing of allanite-based rare earths.
The primary neutralization using 15% MgO for 2 hours at 75oC and pH 3.15 (i.e. test PN12) removed 99.8% iron, 89.0% silica, 92.9% thorium, and 99.4% titanium with an average REE loss for light (“LREE”) and heavy rare earths (“HREE”)2 of 0.6% and 0.8%, respectively. Furthermore, 40.5% aluminum was removed during primary neutralization, which is greater than anticipated. The secondary neutralization (i.e. test SN2) using between 5% and 10% MgO for 2 hours at 75oC and pH of 5.0 removed 99.4% Iron, 96.3% aluminum, 71.0% silica, 98.9% Thorium, and 95.6% Titanium from what was left in the solution after the primary neutralization. An average of 7.6% of LREE and 16.7% of the HREE were precipitated during secondary neutralization. Our technical consultants recommend recycling the solids from secondary neutralization back to leaching to capture the REE for reprocessing.
Why it matters?
Impurity removal testing was performed on leachate solutions prepared from mineral concentrate material collected from four core holes at Halleck Creek as previously released3. The main goal of the neutralization tests is to remove impurities (i.e. non-rare earth elements) from the leach liquor containing the dissolved REEs through precipitation, while minimizing the loss of REEs through co-precipitating alongside the impurities.
Impurity removal is a key step in producing rare earth products from Halleck Creek ore. The tests were completed ahead of schedule and the data received will be used in the mineral processing flow-sheet design for the upcoming Pre-Feasibility Study (“PFS”). Removing non-REE elements from leachate solutions enables the REE to be extracted from solution via solvent extraction and ultimately produce separated rare earth oxides (precursors for rare earths permanent magnets). Iron, silica, aluminum and other deleterious elements can contaminate the solvent extraction process and must be removed from the leachate beforehand. The impurity removal testing demonstrated that these elements can removed from leachate solutions thus providing a highly enriched and clean solution for rare earth product refining. The successful completion of these tests is a major metallurgical processing milestone for Halleck Creek’s allanite based rare earths.
Metallurgical Testing Next Steps
- Hydrometallurgical testing is nearing completion.
- SGS will then create a mixed rare earth oxalate by precipitating the REE with oxalic acid.
- The mixed rare earth oxalate will be calcined to create a mixed rare earth oxide (i.e. the precursor to separated rare earth oxides).
- The mixed rare earth oxide will be re-leached. Cerium oxide is insoluble in the leach reagent and will be filtered out of the new leachate solution. The final leachate solution is then ready for future solvent extraction testing.
ARR expects these final tests to be completed before the end of the year. In parallel, bulk samples from the CSM test pit have been delivered to Fl Smidth, Loesche and Weir (Corem) for comminution optimisation testing which is currently in progress. These results will be reported to the market as soon as they are complete.
Additional Technical Details
Impurity removal testing was performed on leachate solutions prepared from mineral concentrate material collected from four core holes at Halleck Creek as previously released4.
In general, iron, silica, and thorium become insoluble in solutions with pH values between 2.75 and 3.25 and precipitated out. REEs generally remain in solution at these same pH ranges. Therefore, by raising the pH of the leachate solution, iron, silica and thorium can be precipitated and removed via filtration while REE stays in solution. This is called primary neutralization.
Aluminum and uranium generally become insoluble in solutions with pH values between 4.5 and 6.0. REE generally remain in solution at these same pH ranges. Increasing pH of the solution in secondary neutralization, iron, thorium, aluminum, and uranium can be precipitated and removed via filtration from solution.
By performing impurity removal in two neutralization steps, fewer REE are precipitated because the chemical reactions are more controlled. If the pH of the leachate solution was suddenly increased to above 3.5, losses of REE through co-precipitation would occur as a result.
Different reagents react differently with chemical elements in various leachate solutions. SGS performed a comprehensive series of tests to determine which chemical reagents and pH values are most effective on Halleck Creek leachate solutions.
Reagent Selection
SGS, in Lakefield Ontario, tested six leach liquor neutralization reagents for impurity removal from leach solutions including:
- Magnesium oxide (MgO)
- Magnesium carbonate (MgCO3)
- Sodium hydroxide (NaOH)
- Sodium carbonate (soda ash)
- Limestone (calcium carbonate)
- Lime (CaO)
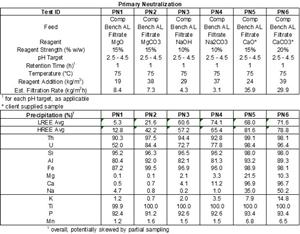
SGS performed pH profile testing over a range of pH values from 2.5, 3.0, 3.5, 4.0, and 4.5 for each reagent, PN1 through PN 6. Table 1 and Figure 1, and Figure 2 summarize the results of the tests. The tests were all performed at 75oC and the reagent strengths varied between reagent types.
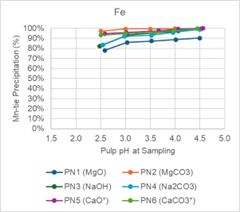
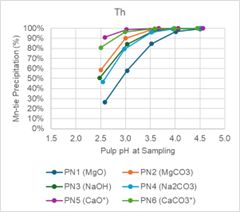
Magnesium oxide and magnesium carbonate performed well in testing. Iron (Fe) and thorium (Th) were precipitated at high levels, while REE precipitation was low across the pH ranges tested. Figure 1 below shows that at pH less than 3.5, Nd and Dy have minimal precipitation. Conversely, Figure 2 shows that Fe and Th have over 80% precipitation when pH is less than 3.5.
The limestone and lime performed poorly because they precipitated gypsum and co-precipitated rare earth elements from the leach solution.
The sodium hydroxide and soda ash also performed poorly because they formed sodium/rare earth double salts and precipitated rare earth elements from the leach solution.
Conversely, to the calcium- and sodium-based reagents, solutions neutralized by MgO did not exhibit formation of gypsum or silica gel during the course of testing. SGS and Tetra tech attribute this to the reagent type, dilution, the temperature of the solutions, and the short residence times of testing.
Table 1 – pH Profile Testing Results for Primary Neutralization by Reagent Type and pH Range
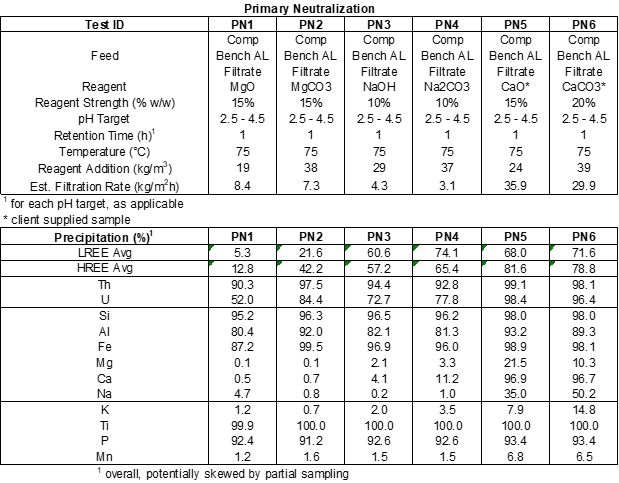
Figure 1 – pH Profiling Charts for Nd and Dy
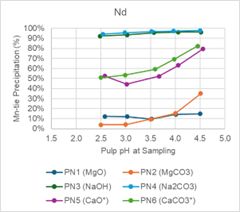
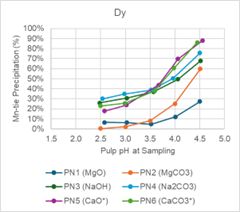
Figure 2 - pH Profile Charts for Fe and Th
Primary Neutralization (“PN”)
The pH profile tests clearly showed that MgO and MgCO3 were superior to the other reagents for impurity removal and rare earth recovery. SGS compared MgO and MgCO3 for primary neutralization at a static pH of 3.25. The tests results were very similar. Tetra Tech engineers determined that MgO is a more cost-effective reagent than MgCO3 when considering dosage rates and the cost of the reagents. Therefore, MgO was selected as the reagent for primary neutralization. It is important to note that both MgO and MgCO3 did not form gypsum or silica gel in the neutralization process. Historically, the formation of these products has proven to be a material technical and economic hurdle to overcome in the processing of allanite-based rare earths.
With the selection of MgO as the neutralization reagent, SGS performed detailed pH endpoint tests for pH ranges from 2.75, 3.0, and 3.25, tests PN7, PN9, and PN10, respectively, see Table 3 and Figure 3. These tests indicate that a target pH of 3.15 is the optimal pH for primary neutralization. Test PN13 was then performed using a pH of 3.15, confirming this value.
Table 3 – Endpoint pH Comparison of Primary Neutralization for MgO
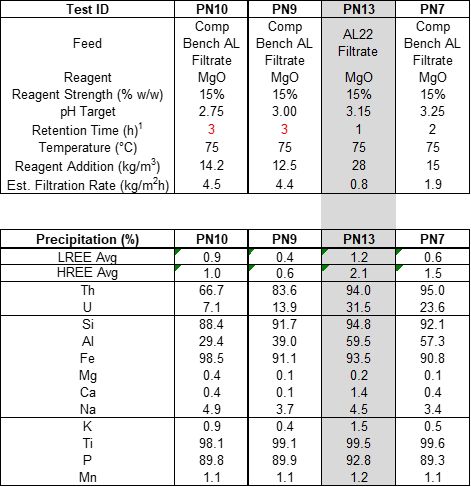
Figure 3 - pH Profile Charts for MgO
SGS performed two additional tests, PN12 and PN14. Tests PN12 and PN14 were conducted using commercially available MgO products near Halleck Creek as a comparison to the locally available MgO used in the other tests.
Test PN13 reduced the residence time of primary neutralization from 2 hours to 1 hour. Reducing the residence time to one hour reduces equipment size and reduces REE losses to precipitation, which ultimately will increase overall rare earth oxide recoveries.
Table 3 – Comparison of Residence Time in Primary Neutralization
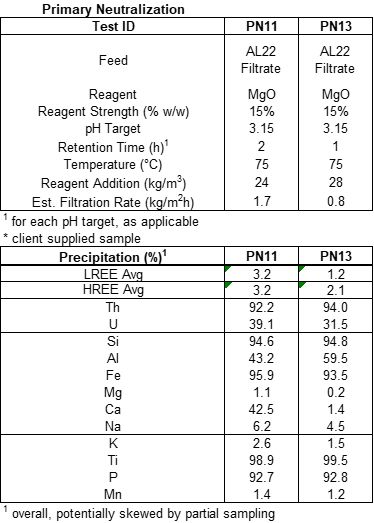
Secondary Neutralization (“SN”)
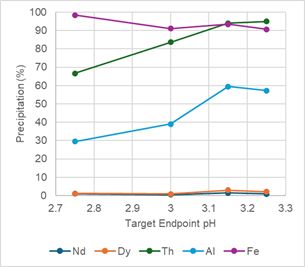
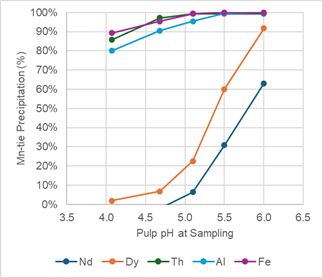
To remove the remaining iron, silica, aluminum, uranium and thorium from solution, SGS performed a pH profile test, SN1, for nominal pH ranges from 4.0, 4.5, 5.0, 5.5 and 6.0, see Table 5. Figure 4 below shows that nearly all the remaining iron, aluminum and thorium are precipitated at a pH near 5.0. Figure 4 also shows that Dy is starting to precipitate at pH 5.0.
Endpoint pH tests were conducted using pH values of 5.0 and 5.25, tests SN2 and SN3, respectively, see Table 5. Based on these two observations, SGS and the ARR team determined that secondary neutralization is best achieved at a target pH of 5.0.
A final leachate solution was prepared by using leachate from test PN11 using the parameters in test SN2. The resulting leach solution will be used for bench scale ion exchange removal of residual uranium and to feed into oxalic acid precipitation to produce a mixed rare earth oxalate. These tests will be completed prior to the end of the year.
Table 5 – Secondary Neutralization Tests
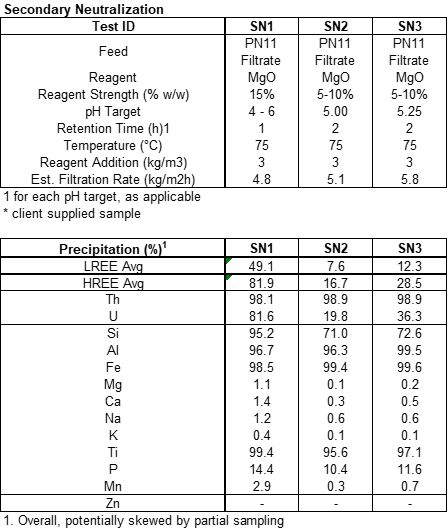
Figure 4 - pH Profile Charts for Secondary Neutralization
It should be noted, to prevent REE losses in the system, SGS and Tetra Tech recommend that the precipitated solids generated during secondary neutralization be recycled to the leach circuit andre-dissolved. While this increases the total volume of material being leached by about 1% or 2%, capturing the REE in this material is most beneficial for the project.
This release was authorized by the board of American Rare Earths.
Investors can follow the Company’s progress at www.americanree.com
The complete JORC Report can be found here:
About American Rare Earths Limited:
American Rare Earths (ASX: ARR | OTCQX: ARRNF | ADR: AMRRY) is a critical minerals company at the forefront of reshaping the U.S. rare earths industry. Through its wholly owned subsidiary, Wyoming Rare (USA) Inc. (“WRI”), the company is advancing the Halleck Creek Project in Wyoming—a world-class rare earth deposit with the potential to secure America’s critical mineral independence for generations. Located on Wyoming State land, the Cowboy State Mine within Halleck Creek offers cost-efficient open-pit mining methods and benefits from streamlined permitting processes in this mining-friendly state.
With plans for onsite mineral processing and separation facilities, Halleck Creek is strategically positioned to reduce U.S. reliance on imports—predominantly from China—while meeting the growing demand for rare earth elements essential to defense, advanced technologies, and economic security. As exploration progresses, the project’s untapped potential on both State and Federal lands further reinforces its significance as a cornerstone of U.S. supply chain security. In addition to its resource potential, American Rare Earths is committed to environmentally responsible mining practices and continues to collaborate with U.S. Government-supported R&D programs to develop innovative extraction and processing technologies for rare earth elements.
For further information contact:
|
Susan Assadi Media Relations US sassadi@americanree.com 347 977 7125 |
Beverly Jedynak Investor Relations US Beverly.jedynak@viriathus.com 312 943 1123 |
1 See ASX Release dated July 16, 2025
2 Light Rare Earths include La, Ce, Pr and Nd. Heavy rare earths include Sm, Eu, Gd, Tb and Dy.
3 ASX Release 16 July, 2025
4 ASX Release 16 July, 2025
Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/08e80a81-df57-4dbc-80d7-e5faa9e7dfd7
https://www.globenewswire.com/NewsRoom/AttachmentNg/4e2d9026-77cc-46e3-a73d-849a948169fc
https://www.globenewswire.com/NewsRoom/AttachmentNg/aac0fe38-559c-4289-b07f-9320cbf3ee41
https://www.globenewswire.com/NewsRoom/AttachmentNg/f21c5df0-9aaa-4bef-ad0a-5712a6c53093
https://www.globenewswire.com/NewsRoom/AttachmentNg/49760699-2f78-4d74-9e36-c720cde48044
https://www.globenewswire.com/NewsRoom/AttachmentNg/22f8f86f-5a23-4a01-bf77-d73d03ef9a35
https://www.globenewswire.com/NewsRoom/AttachmentNg/f72cacbd-11f0-4e72-bc14-92ce461208d6
https://www.globenewswire.com/NewsRoom/AttachmentNg/c5ac1beb-a7c9-44a2-9bf1-24e8dc4cee22
https://www.globenewswire.com/NewsRoom/AttachmentNg/db29e893-c733-4901-a36a-165092b6da67
https://www.globenewswire.com/NewsRoom/AttachmentNg/b1370211-20a0-47d8-b738-33342f963ee9

Table 1 –
pH Profile Testing Results for Primary Neutralization by Reagent Type and pH Range
Figure 1 –
pH Profiling Charts for Nd and Dy
Figure 1 –
pH Profiling Charts for Nd and Dy
Figure 2 -
pH Profile Charts for Fe and Th
Figure 2 -
pH Profile Charts for Fe and Th
Table 3 –
Endpoint pH Comparison of Primary Neutralization for MgO
Figure 3 -
pH Profile Charts for MgO
Table 3 –
Comparison of Residence Time in Primary Neutralization
Table 5 –
Secondary Neutralization Tests
Figure 4 -
pH Profile Charts for Secondary Neutralization
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.